June 2018- Efforts to translate and culturally validate patient-reported health-related quality of life measures are scarce in SSA are scarce. This study translates the PROMIS-25 to Chichewa and assesses its validity and reliability in our setting.
Translation, psychometric validation, and baseline results of the Patient-Reported Outcomes Measurement Information System (PROMIS) pediatric measures to assess health-related quality of life of patients with pediatric lymphoma in Malawi
Westmoreland K, Reeve BB, Amuquandoh A, van der Gronde T, Manthalu O, Correia H, Stanley C, Itimu S, Salima A, Chikasema M, Ward P, Mpasa A, Wachepa S, Mtete I, Butia M, Chasela M, Mtunda M, Wasswa P, Martin S, Kim NE, Kazembe P, Gopal S
Pediatric Blood Cancer
Access the full article here.
Abstract
Introduction
Internationally validated tools to measure patient-reported health-related quality of life (HRQoL) are available, but efforts to translate and culturally validate such tools in sub-Saharan Africa (SSA) are scarce, particularly among children.
Methods
The Patient-Reported Outcomes Measurement Information System 25-item pediatric short form (PROMIS-25) assesses six HRQoL domains-mobility, anxiety, depression, fatigue, peer relationships, and pain interference-by asking four questions per domain. There is a single-item pain intensity item. The PROMIS-25 was translated into Chichewa and validated for use in Malawi using mixed qualitative and quantitative methods. The validity and reliability of the PROMIS-25 was assessed.
Results
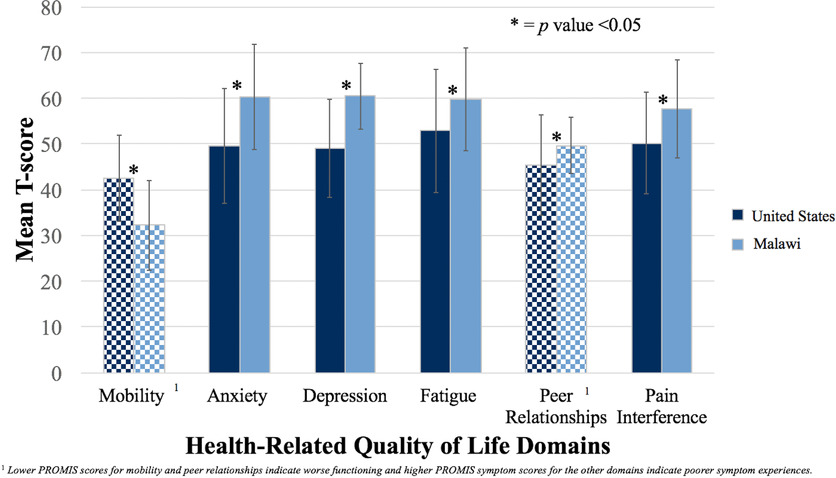
Fifty-four pediatric patients with lymphoma completed the PROMIS-25. Structural validity was supported by interitem correlations and principal component analysis. Reliability of each scale was satisfactory (range alpha = 0.71-0.93). Known group validity testing showed that anemic children had worse fatigue (P = 0.016) and children with poor performance status had worse mobility (P < 0.001) and pain interference (P = 0.005). Compared to children with cancer in the United States, children from Malawi reported lower levels of mobility, higher anxiety, higher depressive symptoms, higher fatigue, better satisfaction with peer relationships, and higher pain interference.
Conclusion
Translation and cultural validation of the PROMIS-25 into Chichewa for Malawi was successful. Baseline HRQoL for patients with pediatric lymphoma in Malawi is poor for all domains except peer relationships. This emphasizes an urgent need to address HRQoL among children undergoing cancer treatment in SSA using self-reported instruments validated within the local context.